Major milestone in Alzheimer's research, experts able to reverse effects
text_fieldsCambridge: Researchers have found a way to reverse the effects of Alzheimer's disease. Tests conducted in mice show that a string of amino acids can interfere with an enzyme that is overactive in the brains of people with the neurodegenerative disease.
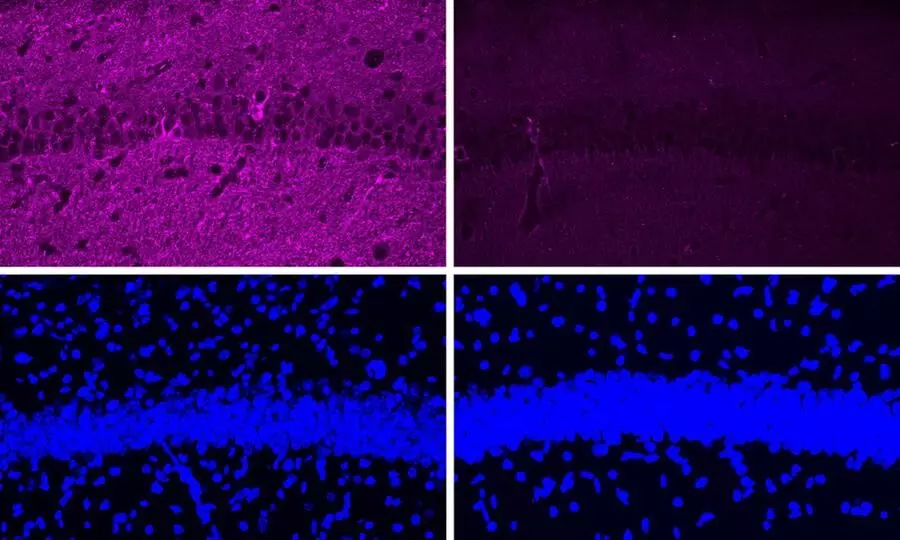
The enzyme CDK5 is typically hyperactive in the brain affected by Alzheimer's disease. Scientists used a peptide to block the hyperactive version of the enzyme which led to dramatic reductions in neurodegeneration and DNA damage in the brain.
It does not interfere with the CDK1 which is an enzyme the human body needs and is structurally similar to CDK5.
A report in New York Post said that the peptide has the ability to enter the brain. "In a couple of different models, the peptide shows protective effects against loss of neurons and also appears to be able to rescue some of the behavioural deficits," said Li-Huei Tsai, director of MIT's Picower Institute for Learning and Memory.
Scientists are hoping to use the peptide as a treatment for patients with forms of dementia that have CDK5 overactivation.
The report released by MIT said that the peptide is similar in size to other peptide drugs that are used in clinical applications. "CDK5 is activated by a smaller protein that it interacts with, known as P35. When P35 binds to CDK5, the enzyme's structure changes, allowing it to phosphorylate - add a phosphate molecule to - its targets."
"In Alzheimer's and other neurodegenerative diseases, P35 is cleaved into a smaller protein called P25, which can also bind to CDK5 but has a longer half-life than P35. When bound to P25, CDK5 becomes more active in cells. P25 also allows CDK5 to phosphorylate molecules other than its usual targets, including the Tau protein. Hyperphosphorylated Tau proteins form the neurofibrillary tangles that are one of the characteristic features of Alzheimer's disease," it added.
Earlier efforts by pharmaceutical firms to target P25 with small-molecule drugs have led to side effects because they interfere with other cyclin-dependent kinases.
The MIT team took a different approach to target P25 and used a peptide instead of a small molecule. The peptide was designed with a sequence identical to that of a segment of CDK5 known as the T loop, which is a structure critical to the binding of CDK5 to P25. The peptide has only 12 amino acids which are slightly longer than existing peptide drugs.
"From a peptide drug point of view, usually smaller is better. Our peptide is almost within that ideal molecular size," said author Tsai.
Researchers observed changes in gene expression occurring in mice with Alzheimer's after the peptide was applied. Around 20 genes typically activated by a family of gene regulators called MEF2 showed changes. These can lead to resilience to cognitive impairment.