Phase 2 trial of Bharat Biotech's intranasal vaccine begins in UP's Kanpur
text_fieldsKanpur (Uttar Pradesh): Hyderabad-based Bharat Biotech, the manufacturer of COVID-19 Covaxin and Washington University School of Medicine in St Louis, have commenced the phase 2 trials of India's first nasal vaccine against COVID-19, at Prakhar hospital in Kanpur.
Kanpur is the only centre in the state where intranasal vaccine trials against Covid are being done.
The Phase 1 clinical trial was completed in the age groups ranging from 18-60 years.
As many as 30 volunteers including doctors, their family members and others have administered the first dose of the intranasal vaccine.
Another 20 people will be administered this vaccine over the next few days. The second dose of the vaccine will be administered after 28 days to the volunteers.
All the volunteers who were administered nasal vaccine are adults.
Principal investigator for intranasal Covid vaccine, Dr J.S. Kushwaha said that the doses of the vaccine administered to healthy volunteers in this phase of trial have been well tolerated and no serious adverse events have been reported. All the volunteers are under observation.
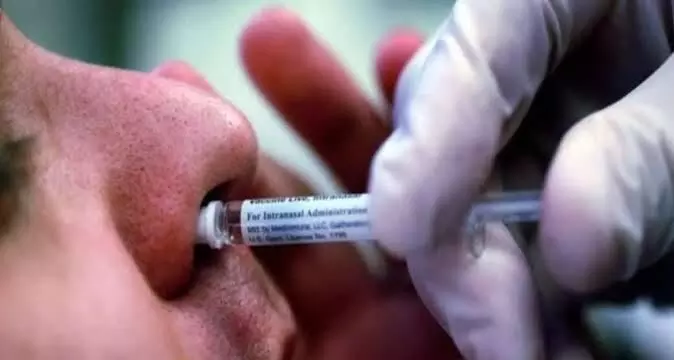
Dr Kushwaha, who administered the vaccine to the volunteers, two drops of the intranasal vaccine were put into each nostril of a volunteer.
After a gap of five minutes two more drops were administered in the same fashion. This way a total of eight vaccine drops was administered to each person. The volunteer was then observed for any reaction for over an hour before being allowed to go home.
Those administered vaccines will now report after 28 days and the same procedure will be repeated.
He also said that prior to the administration of the intranasal vaccine the second time, saliva and blood samples will be collected for studying immunogenicity.
"The intranasal vaccine is dropped into the nostril of a person and it gradually slips into the respiratory tract. The dosage of this vaccine is repeated after 28 days just like Covaxin, which is administered twice in the same time period. The vaccine is designed to neutralise the virus in the nostril itself and prevent it from penetrating deep down into the respiratory tract," he said.