Musk's Neuralink gets approval for human trial in Paralysis patients
text_fieldsElon Musk's brain-chip startup, Neuralink, has received approval from an independent review board to initiate recruitment for its first human trial involving a brain implant designed for paralysis patients.
Individuals with paralysis resulting from cervical spinal cord injuries or amyotrophic lateral sclerosis (ALS) may be eligible to participate in the study.
While the exact number of participants in the trial was not disclosed, the trial is expected to span approximately six years, reported Reuters.
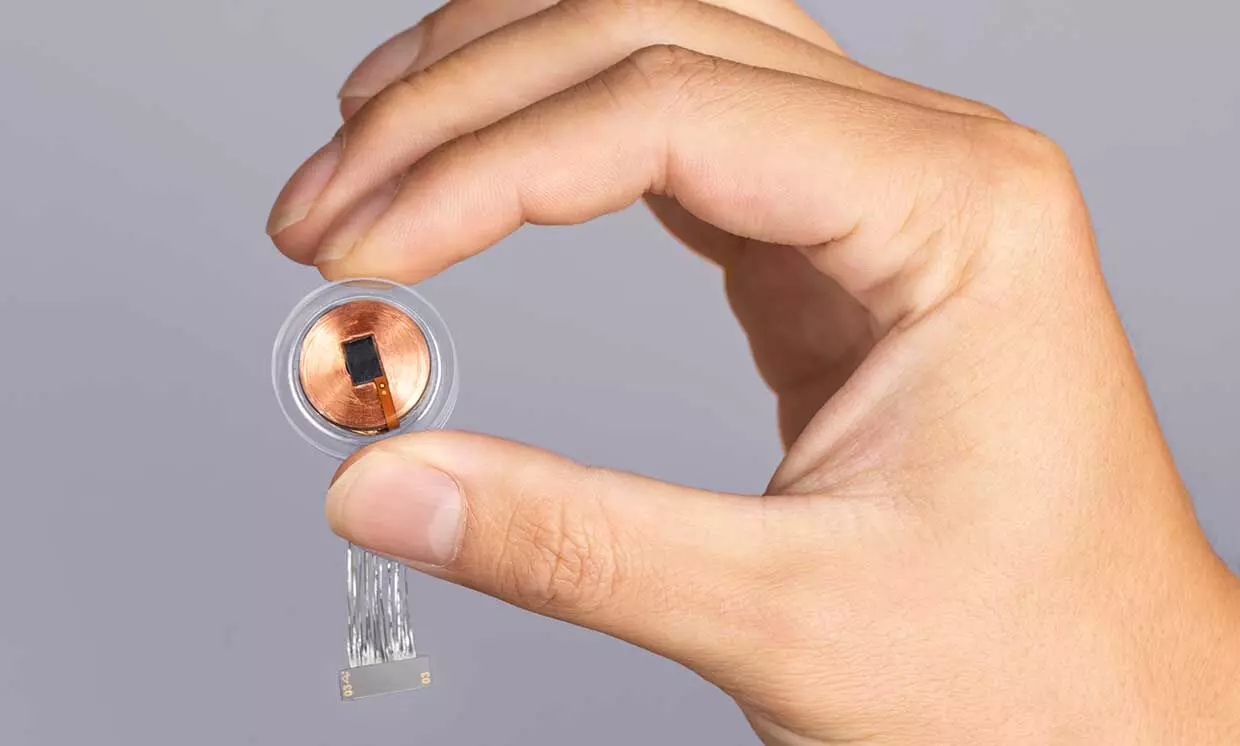
Neuralink's primary objective in this study is to develop a brain-computer interface (BCI) implant that allows individuals to control a computer cursor or keyboard solely using their thoughts.
The procedure will involve the use of a robot to surgically implant the BCI device into a specific brain region responsible for controlling intentional movement.
Initially, Neuralink had sought approval to implant its devices in ten patients; however, negotiations with the US Food and Drug Administration (FDA) led to a lower number due to safety concerns. The exact number of patients approved by the FDA remains undisclosed.
Elon Musk has ambitious plans for Neuralink, envisioning its potential to enable rapid surgical implantation of chip devices for treating conditions such as obesity, autism, depression, and schizophrenia.
While Neuralink has received FDA clearance for its first-in-human clinical trial, it is worth noting that even if the BCI device proves safe for human use, the path to securing commercial clearance is expected to take over a decade, as per expert opinions.
Neuralink's strides in developing brain-computer interfaces signify significant progress in the field of neurotechnology, with potential implications for enhancing the lives of individuals with paralysis and various neurological conditions.